|
|
Equilibrium
Most chemical reactions take place in one direction, from reactants to products (usually written left to right on the page).
The word Equilibrium is the name given to a system with reactions taking place in both directions (left to right and right to left as written)
such that the concentrations of the reactants and products remain constant with time.
Equilibria come in two types:
Homogeneous - Where all of the reactants and products are in the same physical state.
e.g. 2N2(g) + 3H2(g)
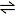 2NH3(g) 2NH3(g)
Where it can be seen that all of the reactants and products are in the gas state.
Heterogeneous - Where some of the reactants or products are in different physical states.
e.g. CaCO3(s) 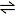 CaO(s) + CO2(g) CaO(s) + CO2(g)
Here the CO2 is present as a gas where the other two substances are present as solids.
The type of equilibrium only affects the mathematics of the equilibrium and not how they respond to changes in conditions.
For more details on the effects of changing conditions on an equilibrium, click on the Equilibrium 1 button to the left.
If you want to find out more about the mathematics of equilibria the the button Equilibrium 2 is for you.
|
|
Here are some other examplesof both homogeneous and heterogeneous equilibria:
|





