|
|
Quantitative Equilibrium
If you remember from the equilibrium page, this relates to a group of reactions we call equilibrium reactions. These occur simultaneously in bot directions, forward and reverse,
at equal rates such that the overall concentrations of reactants and products remain the same. e.g.
Nitrogen + Hydrogen 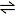 Ammonia Ammonia
2N2(g) + 3H2(g)
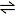 2NH3(g) 2NH3(g)
In such reactions there is a mathematical
relationship between the concentrations of the reactants and products.
In this case it is:
Kc = [NH3(g)]
2 [N2(g)]2[H2(g)]3
Where Kc is called the equilibrium constant (this time where the concentrations are given in moldm-3)
and
[ ] represents the equilibrium concentrations of the reactants and products (measured in moldm-3).
Remember that the terms for the products form the numerator and the reactants the denominator. Each concentration is raised to a power which is the balancing number in the chemical
equation. (Called the stoichiometric coefficient)
|
|
Some examples of Equilibrium Expressions
Below are some examples of equilibria and their Kc expressions:
a).N2O4(g) 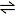 2NO2(g) 2NO2(g)
Kc = [NO2(g)]2
[N2O4(g)]
Units of Kc = moldm-3
b).
|





