|
|
Qualitative Equilibrium
On this page we are going to look at how changes in the conditions that an equilibrium exists at affect it's position. How an equilibrium responds to changes in conditions
is embodied in what is known as "Le Chatelier's Principle".
Henry Le Chatelier was a French chemist who is primarily known for his work on equilibrium reactions.
His principle states that:
If the conditions under which a reaction exists at equilimrium are changed, the equilibrium shifts so as to oppose that change, as far as is possible
(i.e. until something runs out).
The following describes how each change of condition affects an equilibrium and why (always having Le Chatelier's Principle at the back of our minds).
Here we will look at the effects of changing temperature, pressure, concentration and adding a catalyst.
For our
purposes we are going to use the synthesis of ammonia to explain this:
2N2(g) + 3H2(g)
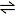 2NH3(g) ΔHf = -92 kJmol-1 2NH3(g) ΔHf = -92 kJmol-1
1). The effect of changing temperature:
How an equilibrium responds to a change in temperature depends on whether the forward reaction is endo- or exothermic.
If the temperature of a system at equilibrium is changed, it will move to oppose that change. So if the forward reaction is exothermic, an increase in temperature
will move it in the reverse endothermic direction as this will use up heat and lower the temperature again. If the temperature is lowered the reverse will occur.
So for our example equilibrium, an increase in temperature favours the reverse, endothermic, reaction increasing the concentrations of nitrogen and hydrogen,
and decreasing the yield of ammonia. The opposite is true for a decrease in temperature.
[Note that although the yield will increase as the temperature is lowered, it will also be formed more slowly.]
2). The effect of changing the pressure:
[This will only affect equilibria where there are gases present and there are different numbers of gas molecules either side of the reaction.]
If the pressure of a system at equilibrium is changed, it will move to oppose that change.
So if the forward reaction involves an increase in the number of gas molecules, an increase in pressure
will move it in the reverse direction as this will favour a decrease in the number of molecules which will reduce the pressure again. If the pressure
is lowered the reverse will occur.
So for our example equilibrium, an increase in pressure favours the forward reaction, as four gas molecules decreases to two, increasing the concentration of ammonia,
therefore increasing the yield of ammonia. The opposite is true for a decrease in pressure.
[Note that an increases in pressure increase the yield and the rate at which it is formed.]
3). The effect of changing the concentration of one of the substances:
|
|
Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem
Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum
Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem
Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum
Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem
Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum
Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem
Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum Lorem Ipsum
Lorem Ipsum |





